| New Search | Back to Search Results |
| | Class 1 Device Recall Colleague 3 and Colleague 3 CX Volumetric Infusion Pumps |
| Date Initiated by Firm | February 25, 2005 |
|---|
| Date Posted | September 23, 2005 |
|---|
| Recall Status1 | Terminated 3 on February 16, 2011 |
|---|
| Z-1544-05 |
|---|
| | Recall Event ID | 31188 |
|---|
| K041191 |
|---|
| infusion pump - Product CodeFRN
|
|---|
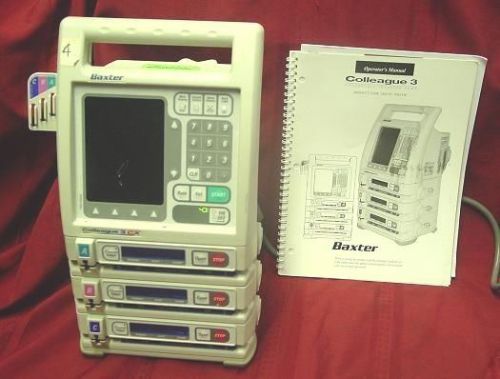
| | Product | Baxter Colleague Triple Channel Volumetric Infusion Pumps; Baxter Healthcare Corporation, I.V. Systems Division, product codes 2M8153, 2M8153R, 2M8163, 2M8163R |
|---|
| Code Information | product codes 2M8153 and 2M8153R: all serial numbers below 13120001CT; product codes 2M8163 and 2M8163R: all serial numbers below 13110338TC |
|---|
Recalling Firm/
Manufacturer | Baxter Healthcare Corp.
Rt. 120 & Wilson Rd
Round Lake IL 60073
|
|---|
| For Additional Information Contact | Center for One Baxter
800-422-9837 |
|---|
| Swelling of the sealed lead-acid batteries in the infusion pump can cause internal pump damage, and excessive battery discharge can damage the batteries if the pump is left on battery power for an extended period of time after teh Battery Depleted alarm occurs. |
|---|
|
| Labeling design |
|---|
| Baxter sent letters dated 02/25/05 to all Colleague infusion pump customers to provide them with important supplemental information to the letter dated 1/21/03 (Z-929-1), regarding sealed lead-acid batteries used in the Colleague family of infusion pumps, dealing with swollen batteries and excessive deep discharge. The 2/25/05 letter reiterated the battery service life, replacement and pump storage conditions which were listed in the 1/21/03 letter, and informed the users that current production pumps have an overcurrent protection circuit in the battery harness to help protect the batteries from overcurrent damage and swelling during charging, and advised them of the availability of the battery harness. The letters also provided information to prevent excessive discharge, and advised them that software updates will become available later in 2005. An Operator's Manual Addendum was also included with the 2/25/05 letter, replacing chapter 7 of the manual dealing with Maintenance and Service. Any questions were directed to Baxter's Medication Delivery Services at 1-800-843-7867. |
|---|
| 53,686 pumps |
|---|
| Nationwide including Puerto Rico, and internationally to Taiwan, Canada, Chile, Korea, Colombia, New Zealand, China, Hong Kong, Brazil, Turkey and Saudi Arabia. |
|---|
| TPLC Device Report |
|---|
1 A record in this database is created when a firm initiates a correction or removal action. The record is updated if the FDA identifies a violation and classifies the action as a recall, and it is updated for a final time when the recall is terminated. Learn more about medical device recalls.
2 Per FDA policy, recall cause determinations are subject to modification up to the point of termination of the recall.
3 For details about termination of a recall see Code of Federal Regulations (CFR) Title 21 §7.55. |
| 510(K) Database | 510(K)s with Product Code = FRN and Original Applicant = BAXTER HEALTHCARE CORP.
|
|---|
When people are very sick, they still need help ingesting medications and nutrients that help their bodies maintain basic balance and function. For many years, these patients had to undergo regular infusions that were manually administered by medical personnel, and this reality prompted companies to formulate products that automated this process. One of the leading companies in this regard, Baxter International, released a line of products several years ago that are known as Colleague Infusion pumps that are designed to deliver medications and nutrients to the patients who need them.
Unfortunately for thousands of people, this line of Baxter Colleague Infusion pumps has been under intense scrutiny for many years because of a large number of reported adverse effects after patients used them. This has led to the decision by the FDA to order a recall of these Baxter Colleague Infusion pumps. Below youll find information regarding a description of these products, the problems they caused, the governmental involvement with these issues and finally how you should proceed if you or someone you love has been harmed by the use of a Baxter Colleague Infusion pump.
Baxter Colleague Infusion Pumps A Brief Overview
Baxter International is the corporate umbrella that manufactures and distributes the entire line of Colleague Infusion pumps around the world. In terms of the United States, these products are the responsibility of the Baxter Healthcare Corporation, which is the American subsidiary of the company. https://generatornin.netlify.app/roland-ep-75-digital-piano-user-manual.html.
These products have been in circulation for several years, and have been used to deliver nutritional substances and medications to patients who are in comas and have other health problems and have been used in hospitals and even in homes. Estimates indicate that despite the fact that no new models have been offered to the market since 2005, there are still an estimated 200,000 units currently in use.
Adverse Effects to Baxter Colleague Infusion Pumps
The history of adverse effects while using these products dates back as far as 1999. The specific problems associated with these pumps include the following:

- Software defects
- User interface difficulties
- Mechanical failures
- Overheating problems
- Electrical failures
- Battery failures

All of these problems have led to one basic result the unforeseen interruption of therapy that basically stops the delivery of necessary nutrients and medication to patients who need them to survive. As a result of these ongoing problems, there have been reports of as many as 56,000 adverse effects while using these products and upwards of 500 deaths.
FDA Involvement with Baxter Colleague Infusion Pumps
As the number of reports of adverse effects continued to rise, the United States Food and Drug Administration (FDA) began to get involved with the regulation of these products in an effort to determine whether or not to require changes to them and/or a recall of them if their overall performance record did not improve.
This process by the FDA began in 1999, and since then there have been three separate Class I recalls, which is the most serious and dangerous type of recall in existence thats only used when users of a product or medication are at risk for serious health consequences and death. These recalls took place in 2005, 2009 and May of 2010. The models affected by these recalls include the models labeled Mono, CX & CXE.
British household panel survey user manual free. The survey is funded by the Australian Government through the.HILDA has the following key features:.
Based on the ongoing dealings between Baxter and the FDA, several offers by the company to improve the performance of these models have been proposed. The latest proposition offered by Baxter stated that it would begin the process of making the necessary corrections to these models in May of 2012, with a hoped-for date of completion of these corrections to these defective Baxter Colleague Infusion pumps falling in 2013.
As would be expected, the FDA did not approve of this proposal given that the result would be that thousands of defective Baxter Colleague Infusion pumps would remain on the American market for at least three more years until even the most basic safety concerns could be considered as properly addressed.
Blood Pressure mode: current blood pressure monitoring, switch the key to the blood pressure interface, measurement mode will open, if not, please continue switching, bracelet appears as the most recent measurement data. https://generatornin.netlify.app/intelligence-health-bracelet-m3-user-manual.html. Mobile applications display data by A single measurement, real-time measurement or 1: key measurement. HR intertace, pls let the skin wrist above be close to the bracelet to prevent light leakage.7.
Therefore, the FDA has issued a statement requiring the immediate recall of Baxter Colleague Infusion pumps. While this step could help with the overall safety of the public that uses them for critical life-maintaining treatments, it does nothing to help those who have already been harmed as a result of using these products.
How a Defective Medical Devices Lawyer Can Help
If you or someone you love has been harmed by the use of any of the Baxter Colleague Infusion pumps that either were or currently are on the market, you need to take immediate action to protect and enforce your legal rights. Contact the defective medical device lawyers at Phillips Law Group immediately to schedule a free initial consultation.
Skech bluetooth headphones bth561 user manual. The headset uses Bluetooth technology, meaning you can talk on the phone or listen to music wirelessly, so there are no messy cables to deal with. You can even control your device directly from the headset with the built-in controls, making it easy to play, pause, or skip tracks and even command Siri.
Related Topics - Defective Products
- DePuy Hip Replacement Implant
Baxter Infusion Pump


